What is Calcium Hypochlorite?
Calcium Hypochlorite │ Ca (ClO)2 is a chemical compound that has the formula Ca (ClO)2.
It is commonly referred to as bleaching powder or calcium oxychloride. It is an ionic compound that is made up of a calcium cation (Ca2+) and two hypochlorite anions (ClO–). Despite being quite stable at room temperatures, calcium hypochlorite slowly decomposes in moist environments, giving it a characteristic ‘chlorine’ smell.
Calcium Hypochlorite Preparation
It is commercially available in its anhydrous and hydrated forms and is one of the key ingredients of chlorine powder and bleaching powder. Ca(ClO)2 is produced on an industrial scale via the reaction of chlorine gas with calcium hydroxide.
The chemical equation for this reaction is provided below.
2Ca (OH)2 + 2Cl2 → Ca (ClO)2 + H2O + CaCl2
Generally, bleaching powder is a mixture of calcium hypochlorite, its dibasic form (Ca(ClO2).2Ca(OH)2), and the dibasic form of calcium chloride (CaCl2.2Ca(OH)2). Since it contains two ClO– ions, this compound has a high chlorine availability when compared to sodium hypochlorite (NaOCl).
Calcium Hypochlorite Structure
The structure of a Ca (ClO)2 molecule is illustrated below. It consists of one Ca+ ion and two ClO– ions.

Structure of a Calcium Hypochlorite Molecule
Each chlorite ion has a charge of -1 whereas the calcium ion has a charge of +2. Therefore, one calcium cation can form ionic bonds with two hypochlorite ions.
Calcium Hypochlorite Properties
1. Chemical Data
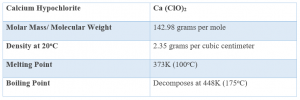
2. Physical Properties
- Calcium hypochlorite is a white/grey solid at room temperatures.
- Its solubility in water is 21g/100 mL and it reacts with the water when dissolved.
- Its solubility in hard water is relatively low when compared to its solubility in soft/medium-hard water.
- Ca (ClO)2 has a strong smell of chlorine (because it reacts with the water molecules present in air).
3. Chemical Properties
- It acts as a strong base since it readily accepts H+ When dissolved in water, the hypochlorite anion accepts a proton from H2O, liberating an OH– ion.
- The chemical reaction is given by ClO– + H2O → HClO + OH–
- This compound is also a powerful oxidizing agent since it can readily accept an electron.
- Calcium hypochlorite reacts with hydrochloric acid, yielding calcium chloride (CaCl2), water, and chlorine gas (Cl2).
- The chemical equation for this reaction is
4HCl + Ca(ClO)2 → CaCl2 + Cl2 + H2O
What are the Uses of Calcium Hypochlorite?
The compound Ca(ClO)2 is often used to disinfect large volumes of water in order to make it safe to drink. It is also widely used in swimming pools to sanitize the water body and destroy the germs present in it. Some other uses of calcium hypochlorite are listed below.
- Since it is a good oxidizing agent, this compound is quite useful in the field of organic chemistry.
- It is used to obtain fragmented aldehydes/carboxylic acids by cleaving the bonds in glycols and keto acids.
- Ca (ClO)2 can also be used in the halo form reaction to yield chloroform.
- The compound can be used to disinfect both wastewater and drinking water since it has a high chlorine availability.
To learn more about calcium hypochlorite and other important calcium compounds, such calcium hydroxide, register with BYJU’S and download the mobile application on your smartphone.
Frequently Asked Questions – FAQs
Q1
Is calcium hypochlorite dangerous?
The corrosive nature of the hypochlorite moiety makes calcium hypochlorite a dangerous substance that must be stored carefully. It must be stored in a cold, dry environment and must not be exposed to any metals or acids. If any acid comes in contact with calcium hypochlorite, highly toxic fumes of chlorine gas can be produced.
Q2
What is the common name of calcium hypochlorite?
Calcium hypochlorite is commonly known as bleaching powder. It is also known as calcium oxychloride, the chloride of lime, or the calcium salt of hypochlorous acid.
Q3
Is calcium hypochlorite soluble in water?
Yes, calcium hypochlorite is fairly soluble in water. Since it is an ionic compound, Ca (ClO)2 readily dissolves in water and dissociates into its constituent ions. At a temperature of 25oC, the solubility of this compound in water is roughly equal to 210 grams per litre. It is important to note that this compound is not soluble in alcohol since it participates in a chemical reaction with the solvent in such cases.
Q4
What is calcium hypochlorite used for?
Calcium hypochlorite is known to be the active ingredient in many commercial bleaching agents such as bleaching powder, chlorinated lime, and chlorine powder. The most important application of calcium hypochlorite is in the sanitization of public swimming pools. It is also used to disinfect drinking water. As an oxidizing agent, calcium hypochlorite is also used in several organic reactions.
Q5
How is calcium hypochlorite produced industrially?
On an industrial scale, calcium hypochlorite is usually produced from the chemical reaction between gaseous chlorine and calcium hydroxide (usually used in the form of the mineral lime). The chemical reaction between calcium hydroxide and chlorine gas yields calcium hypochlorite, calcium chloride, and water as the products.


















